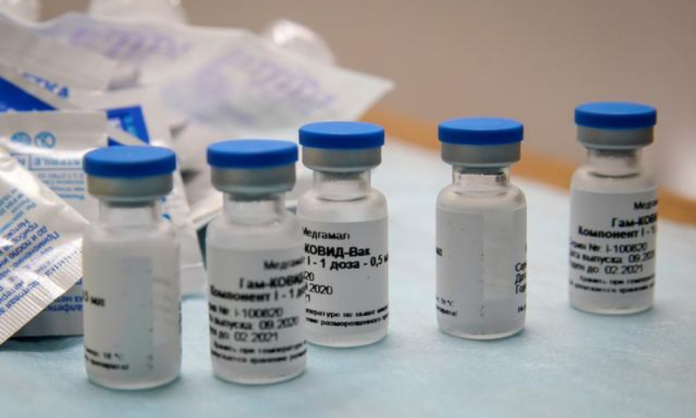
LAHORE: Karachi-based pharmaceutical firm AGP Limited has denied media reports that it had entered into an agreement with any hospital or diagnostic centre for the supply of Russian Sputnik-V vaccine for Covid-19.
Being the sole distributor of Sputnik-V vaccine in Pakistan, AGP Limited on Monday termed the reports circulating on social and electronic media regarding the availability of Sputnik-V vaccine through one of the prominent diagnostic centres in Pakistan as “false, incorrect and misleading.”
“It is hereby clarified that AGP Limited, so far has not entered into a supply agreement with any hospital or Diagnostic Centre, including the Diagnostic Centre in question, for the supply of Sputnik-V vaccine either directly or indirectly in Pakistan,” the company said in a statement.
The pharmaceutical company further added that being the exclusive distributor of Sputnik-V vaccine in Pakistan, they will only enter into a supply agreement with a hospital or diagnostic centre after completion of all necessary legal formalities and which duly conforms and fully complies with the directions issued by the relevant regulatory authorities.
On February 13, Lahore-based Chughtai Lab CEO Omar Chughtai had tweeted: “We are expecting our first shipment of the #COVID vaccine insha Allah within the next 1-2 weeks. The Sputnik V vaccine has been shown to be 91.6pc effective. Already authorised for use in Pakistan.”
“Working on appointment setting system. More details soon,” the CEO further said in the tweet.
At @ChughtaiLab we are expecting our first shipment of the #COVID vaccine insha Allah within the next 1-2 weeks.
The Sputnik V vaccine has been shown to be 91.6 % effective. Already authorized for use in Pakistan.
Working on appointment setting system. More details soon. pic.twitter.com/2kJUzgKvne
— Dr Omar Chughtai (@OmarChughtai) February 13, 2021
However, on February 14, in another tweet, Omar annulled his earlier message that the Sputnik-V vaccine would be available at Chughtai Labs within 1-2 weeks.
“Our vaccination programme will commence after relevant registration and authorizations from Ministry of Health, NIH, DRAP and National Immunization Management System,” he tweeted on Sunday. “At @ChughtaiLab we have always worked within the rules and regulations of relevant health authorities.”
At @ChughtaiLab we have always worked within the rules and regulations of relevant health authorities.
Our vaccination program will commence after relevant registration and authorizations from Ministry of Health, NIH, DRAP and National Immunization Management System.
— Dr Omar Chughtai (@OmarChughtai) February 14, 2021
In a notice issued to the Pakistan Stock Exchange on February 2, the pharmaceutical company AGP said that Drug Regulatory Authority of Pakistan (DRAP) had granted the emergency use of Sputnik-V vaccine against Covid-19, also known as SARS-CoV-2 virus.
The company, AGP Limited, has also been authorised to import and introduce the vaccine and is now making efforts to ensure availability of sufficient supplies on an emergency basis, in order to play a key role in supporting the government’s objective of vaccinating the masses.
According to the AGP, the vaccine was developed by the Gamalama National Center of Epidemiology and Microbiology, Russia, one of the world’s leading research institutions. This vaccine has been approved by fifteen countries, including Russia, Hungary, Argentina and the UAE.
As per the interim clinical study report which is based on the Phase III trials of 21,862 volunteers, the efficacy of Sputnik V has been determined at 91.6pc with 10pc efficacy against severe cases of Covid-19.
“This is by far the most effective Covid-19 vaccine to have been received EUA in Pakistan, and can be administered to a person aged 18 and above, including persons aged above 60,” the AGP notice to the PSX read.